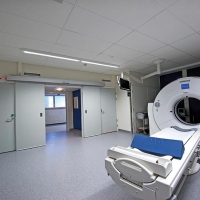
LAMI doors for Pharmaceutical, Laboratories and Cleanrooms
Within pharmaceutical applications, laboratories and cleanrooms the requirements for cleanability and standards of hygiene are extremely high.
LAMI doors are free of timber or other organic materials that promote the bacterial growth. The encapsulated door structure and smooth surfaces of LAMI doors make them extremely easy to clean and will not harbor dirt or bacteria. The surfaces can also withstand the strongest disinfectants and cleaning agents.
LAMI doors product range
LAMI doors product range includes Single Action Doors, Double Action Doors and Sliding Doors. LAMI product range includes also Fire Rated Doors, Acoustic Doors and X-Ray Doors. Fire Rated doors (F240, F120, F90, F60, F30), Classified Acoustic Doors (sound reduction index up to Rw dB35) as well as X-Ray doors (1-4mm lead rate) compliment the features and benefits of the standard LAMI doors in terms of hygiene, durability and aesthetics.
Good Manufacturing Practice (GMP) requirements
We follow Good Manufacturing Practice (GMP) requirements on our all products as detailed in EudraLex - Volume 4 Good Manufacturing practice (GMP) Guidelines and in its Annex 1 chapter 46 of Manufacture of Sterile Medicinal Products.